Immunotherapy for Bile Duct Cancer & Gallbladder Cancer, Stage III NSCLC & Extensive Stage SCLC – IMFINZI® (durvalumab)
US FDA Grants Imfinzi Priority Review For Treatment Of Extensive-Stage Small Cell Lung Cancer - Thailand Medical News

Who Benefits the Most From Adjuvant Durvalumab After Chemoradiotherapy for Non-small Cell Lung Cancer? An Exploratory Analysis - Practical Radiation Oncology

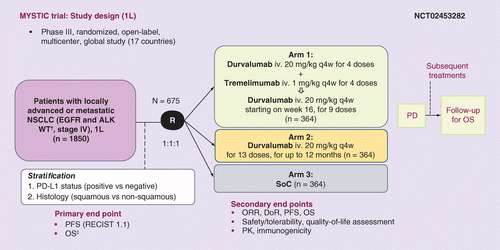
Phase III, randomized, open-label study of durvalumab (MEDI4736) in combination with tremelimumab or durvalumab alone versus platinum-based chemotherapy in first-line treatment of patients with advanced/metastatic NSCLC: MYSTIC | Journal for ...
Yale Cancer Center Study Shows New Drug Combinations Improve Outcomes for Patients With Advanced Lung Cancer < Yale School of Medicine

Four-Year Survival With Durvalumab After Chemoradiotherapy in Stage III NSCLC—an Update From the PACIFIC Trial - Journal of Thoracic Oncology

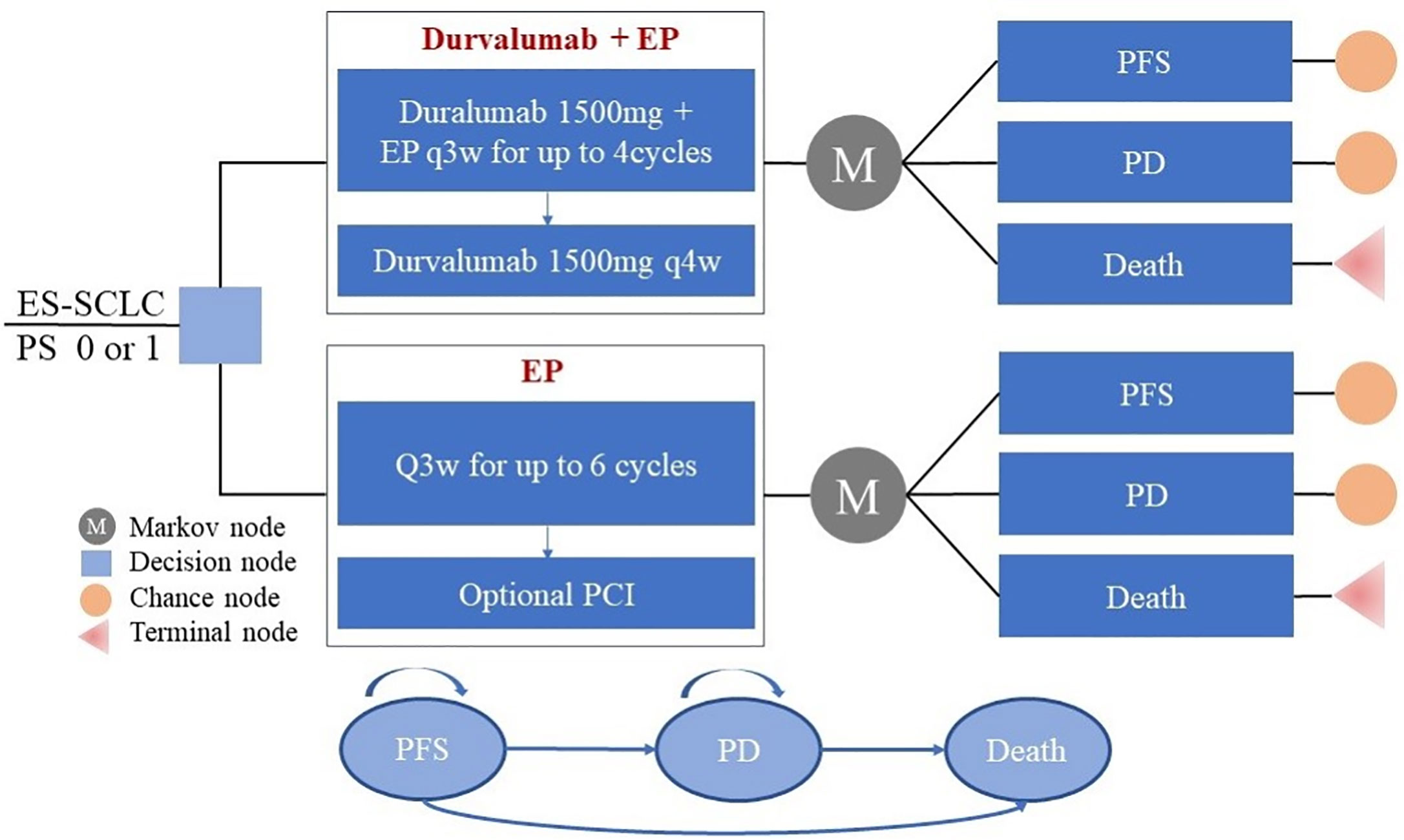
Durvalumab, with or without tremelimumab, plus platinum–etoposide versus platinum–etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial ...

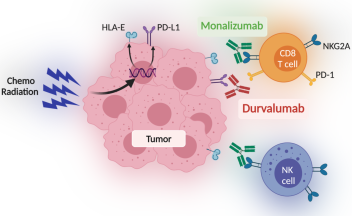
Mechanism of durvalumab. Notes: Durvalumab antibody blocks PD-1 and... | Download Scientific Diagram

Design and Rationale for a Phase III, Double-Blind, Placebo-Controlled Study of Neoadjuvant Durvalumab + Chemotherapy Followed by Adjuvant Durvalumab for the Treatment of Patients With Resectable Stages II and III non-small-cell Lung

IMFINZI® (durvalumab) Plus Chemotherapy Approved in the US as the First Immunotherapy Regimen for Patients with Advanced Biliary Tract Cancer

Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: a single-centre, randomised phase 2 trial - The Lancet Oncology